Greenhouse gases
CSIRO Atmospheric Research Greenhouse Information
Paper
Sunlight passes through the atmosphere, warming the Earth’s surface.
In turn, the land and oceans release heat, or infrared radiation, into
the atmosphere, thus balancing the incoming energy. Water vapour, carbon
dioxide and some of the other trace gases can absorb part of this radiation,
allowing it to warm the lower atmosphere, while the remainder is emitted
to space.
Atmospheric trace gases that trap heat are known as greenhouse gases.
About three-quarters of the natural greenhouse effect is due to water
vapour. The next most significant greenhouse gas is carbon dioxide.
Higher concentrations of carbon dioxide and other greenhouse gases in
the atmosphere will lead to increased trapping of infrared radiation.
The lower atmosphere is likely to warm, changing weather and climate.
Carbon dioxide, methane, nitrous oxide, ozone, synthetic halocarbons
(such as chlorofluorocarbons and hydrofluorocarbons) and sulfur hexafluoride
are all greenhouse gases produced or influenced by human activities.
Carbon dioxide
Carbon dioxide concentrations have increased by 30% during the past 200
years. The major anthropogenic sources of carbon dioxide emissions are
fossil fuel combustion and land clearing for agriculture.
Currently, about 6-8 billion tonnes of carbon (as carbon dioxide) are
emitted during the combustion of fossil fuels and 1-2 billion tonnes from
land clearing. About 3 billion tonnes of the carbon stays in the atmosphere,
while the ocean takes up about 2-3 billion tonnes. Terrestrial sinks,
including greater photosynthesis in plants, take up the remaining 2 billion
tonnes.
(See CSIRO Atmospheric Research Greenhouse Information Paper, The carbon
cycle)
Methane
Atmospheric methane concentrations have increased by 150% during the
past 200 years. The major anthropogenic sources of methane are cattle,
rice growing and leakages during natural gas production, distribution
and use.
During the past century, methane concentrations in the atmosphere have
responded to the growth in global population, in energy use and in agriculture.
Concentrations grew very rapidly during the 1970s and early 1980s, followed
by a slower growth in the 1990s. These changes may, in part, be due to
massive releases of methane from the post World War II natural gas industry
of the former Soviet Union, which were reduced significantly during the
1990s.
Presently, natural processes remove methane from the atmosphere at almost
the same rate as it is being added to it. However, over the next 100 years,
methane concentrations are likely to rise.
Nitrous oxide
Atmospheric nitrous oxide concentrations have increased by 15% during
the past 200 years. Major sources of nitrous oxide include industrial
processes, fertiliser use and other agricultural activities, including
land clearing.
The relative contributions of the various sources to the amount of nitrous
oxide released into the air are uncertain, mainly because there is little
information on land use change in the tropics, which is probably the major
source of this gas.
Ozone
Up to the present, the observed loss of stratospheric ozone (due to the
presence of ozone-depleting substances) has contributed to a cooling of
the atmosphere, which is approximately balanced by the warming due to
the calculated increase in tropospheric ozone (due to air pollution and
photochemical smog) over the past 200 years. Over the next 100 years,
both stratospheric and tropospheric ozone concentrations are likely to
increase.
Halocarbons and sulfur hexafluoride
Atmospheric concentrations of the ozone-depleting halocarbon species
(for example CFCs) are either decreasing or increasing more slowly. These
changes are due to the Montreal Protocol, which is an international agreement
designed to stop ozone depletion.
Concentrations of CFC replacements (such as HFCs), less potent greenhouse
gases than CFCs, are increasing.
Post-industrial revolution warming due to halocarbons and sulfur hexafluoride
is currently largely the result of CFC growth in the atmosphere, but by
the end of the 21st century it may be 75% greater than it is now, largely
due to the growth of HFCs.
HFCs, PFCs (perfluorocarbons, another CFC substitute) and sulfur hexafluoride
(a gas used for electrical insulation) are powerful greenhouse gases,
whose future emissions will be controlled by the Kyoto Protocol. Technologies
exist to reduce emissions of these gases to near zero over the next few
decades. Thus, they represent probably the most significant, immediate
opportunity to slow down the current growth of greenhouse gases in the
atmosphere.
Table. The contributions that carbon dioxide, methane, nitrous oxide,
ozone and synthetic halocarbons/sulfur hexafluoride make, and are likely
to make, to additional atmospheric heat trapping since pre-industrial
times for the years 2000 and 2100.
| |
2000
% |
2000
W/m2
|
2100
% |
2100
W/m2 |
| carbon dioxide |
62 |
1.5 |
63 |
5.9 |
| methane |
21 |
0.5 |
13 |
1.2 |
| nitrous oxide |
4 |
0.1 |
5 |
0.5 |
| total ozone |
0 |
0.0 |
13 |
1.2 |
stratospheric
|
[-16] |
[-0.4] |
[-1] |
[-0.1] |
tropospheric
|
[16] |
[0.4] |
[14] |
[1.3] |
| halocarbons and sulfur hexafluoride |
12 |
0.3 |
6 |
0.5 |
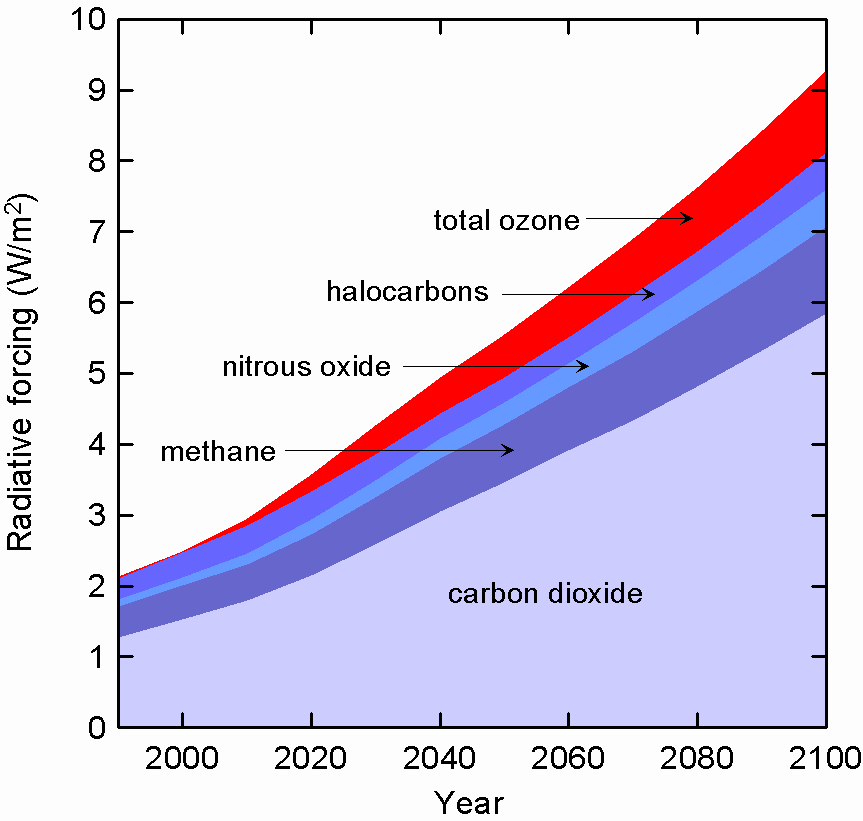
Figure: The change in atmospheric heat trapping (radiative forcing, W/m2)
over the 21st century since pre-industrial times due to long-term growth
in carbon dioxide, methane, nitrous oxide, halocarbons (including sulfur
hexafluoride) and changes to ozone concentrations.
Water vapour
The amount of water vapour humans have added to the atmosphere is not
expected to make a large direct contribution to the enhanced greenhouse
effect. However, warming due to increases in other greenhouse gases is
likely to increase evaporation from the ocean and therefore the amount
of water vapour in the atmosphere, adding to global warming.
December 2000
|

![]()
